Coronary artery in-stent restenosis (ISR) is observed in about 10 percent of patients who undergo a drug eluting stent (DES) implantation and in more than 30 percent of patients who undergo balloon catheter bare-metal stent (BMS) implantation. Three DEBs for the coronaries have now been granted FDA breakthrough device designation to help expedite the approval process.
In July 2020, Med Alliance began enrollment for a study of its Selution SLR 0.014 DEB for the treatment of coronary artery in-stent restenosis (ISR). It was the first DEB accepted by the FDA for its breakthrough program to treat coronary arteries. The Selution SLR (Sustained Limus Release) is a sirolimus-eluting balloon that provides a controlled sustained release of drug, similar to a DES.
The objectives of this prospective, randomized, single-blind multicenter study is to demonstrate the safety and efficacy of Selution SLR in treatment of ISR with either drug-eluting or BMS. The study will support submission for FDA approval. Up to 418 subjects will be recruited into the study at approximately 60 sites across both the U.S. and Europe.
The primary endpoint for effectiveness of the study is target lesion failure (TLF), defined as all cardiac death, target vessel myocardial infarction, or clinically driven target lesion revascularization (TLR) at 12 months. Patients will be followed out to five years.
“No coronary drug-eluting balloon has yet been approved in the U.S., where ISR currently represents 11 percent of all stent implantations,” explained Jeffrey Jump, Med Alliance chairman and CEO.
The balloon uses Micro Reservoirs made from biodegradable polymer, which provide controlled and sustained drug release.
Selution SLR was awarded European CE mark approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary arterial disease in May 2020.
In August 2019, B. Braun Interventional Systems was also granted breakthrough device designation for the SeQuent Please ReX drug-coated percutaneous transluminal coronary angioplasty (PTCA) balloon for the treatment of coronary in-stent restenosis. SeQuent Please ReX is the latest-generation of the European-approved coronary DCB. The DCB has 10 years’ worth of clinical study evaluations.
“We need to have a coronary DCB in our toolbox to treat patients,” said Jorge Saucedo, M.D., chief of cardiology at the Medical College of Wisconsin, “This breakthrough device designation brings the technology a step closer for our use.”
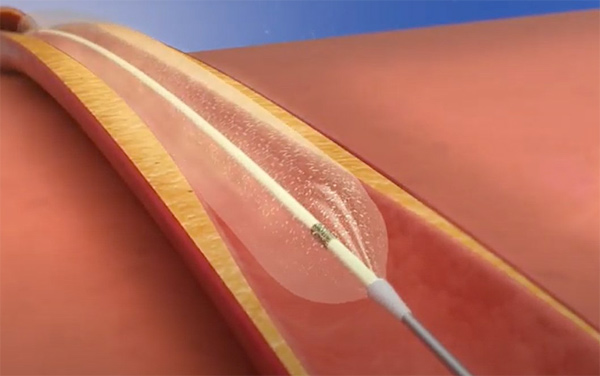
In May 2019, the Concept Medical Inc. MagicTouch sirolimus DCB was granted the breakthrough device designation for the treatment of coronary ISR. The MagicTouch has European CE mark approval. It features a drug delivery technology platform coated onto the balloon designed to deliver sub-micron particles of sirolimus that are then encapsulated in a biocompatible drug carrier. The drug and carrier complex are designed to reach the inner layers of the vessel walls and act as a reservoir for long-term release of sirolimus.
In Europe, iVascular launched its Essential Pro, a coronary artery DCB in June 2020. It offers improvements over the first-generation device with faster deflation times due to an increase of the inflation channel and the availability of two different shafts. It also has improved push ability due to an improved transition lumen structure that transmits the whole push to the distal shaft. Metallic radiopaque markers also improve visibility.
The Essential Pro is indicated for the dilatation of stenosis or coronary artery or bypass grafts occlusions, including small vessels, as well as for residual stenosis after treatment with balloon or endoprosthesis and pre- and post-dilation of coronary endovascular prosthesis.
