On March 14, 2025, Seledora® Coronary Scoring Balloon Catheter, developed by Mixin Medtech(Suzhou) Co., Ltd., a subsidiary of Kossel Medtech (Suzhou) Co., Ltd., received approval from the National Medical Products Administration (NMPA) for market launch (Registration No. 20253030586). Kossel’s independently developed Octoparms® II Vena Cava Filter has also been approved by the NMPA today (Registration No. 20253130576).
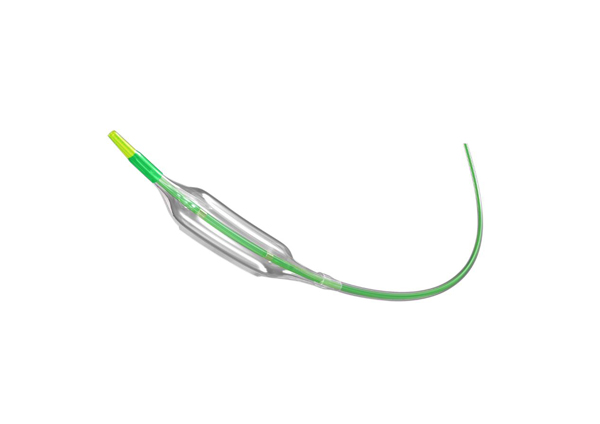
The approval of the Seledora® Coronary Scoring Balloon Catheter marks another breakthrough for Kossel in the full-cycle management of “precise pre-treatment – vascular function restoration” in coronary interventions. Moving forward, Kossel will continue to drive innovation, providing high-quality medical devices to clinical practitioners and developing systematic solutions for coronary interventions.
